Viability and Proliferation assays
The cell responsible for the matrix deposition and contraction in all fibrotic diseases is the myofibroblast and surgically excised specimens from patients with Dupuytren's disease provide an abundant supply of material to develop assays that can be applied to other fibrotic conditions where primary early disease stage human tissues are less readily available. Myofibroblasts are collagen‐producing contractile cells found in normal wound healing and numerous pathologies. Proliferation of myofibroblasts and myofibroblast precursors is important in fibroproliferative tissues, therefore understanding myofibroblast proliferation and differentiation is important in normal tissue remodeling and in fibro-proliferative pathologies, such as Dupuytren's contracture.
We have developed several assays to screen the effects of the probe set on myofibroblast viability and proliferation. Viability was measured using the fluorescence based PrestoBlue® assay. Further direct cell counting was also used as a measure of proliferation using Hoechst 33342, a cell-permeant nuclear counterstain that emits blue fluorescence when bound to dsDNA and quantified directly in situ using Celigo image cytometer.
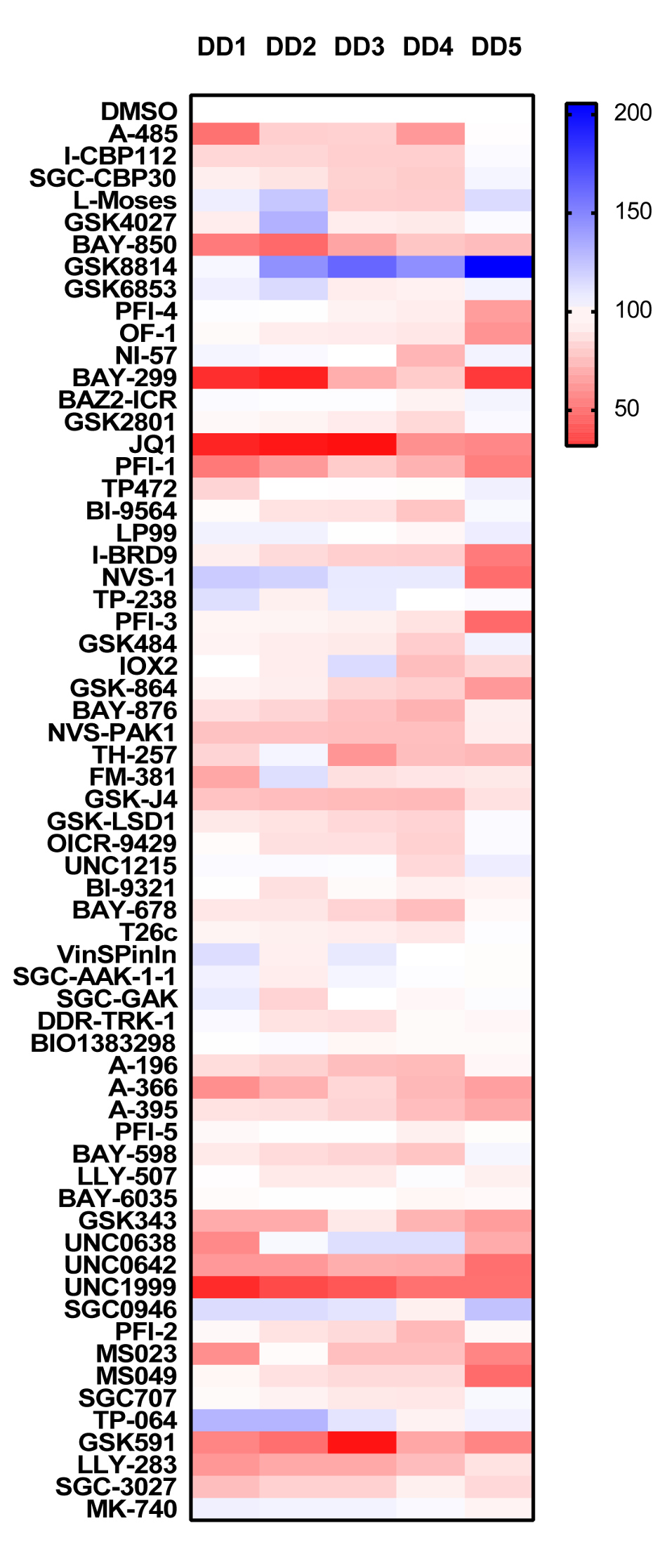
Analysing the PrestoBlue® cell viability data set, the Acetyltransferase probe A485 appears to be significantly toxic, unlike the bromodomain probes SGC-CBP30 and I-CBP112 affecting the same targets (EP300/CREBBP). Other probes targeting bromodomains which also showed significant toxicity were the ATAD2 probe BAY-850, the BRD1 probe BAY-299 and the BRD4 probes JQ1 and PFI-1. The only other probes showing significant levels of toxicity are the methyltransferase inhibitors A-366 (G9a/GLP), GSK343 (EXH2), UNC0638 (G9a/GLP) and UNC0642 (G9a/GLP) and UNC1999 (EXH2).
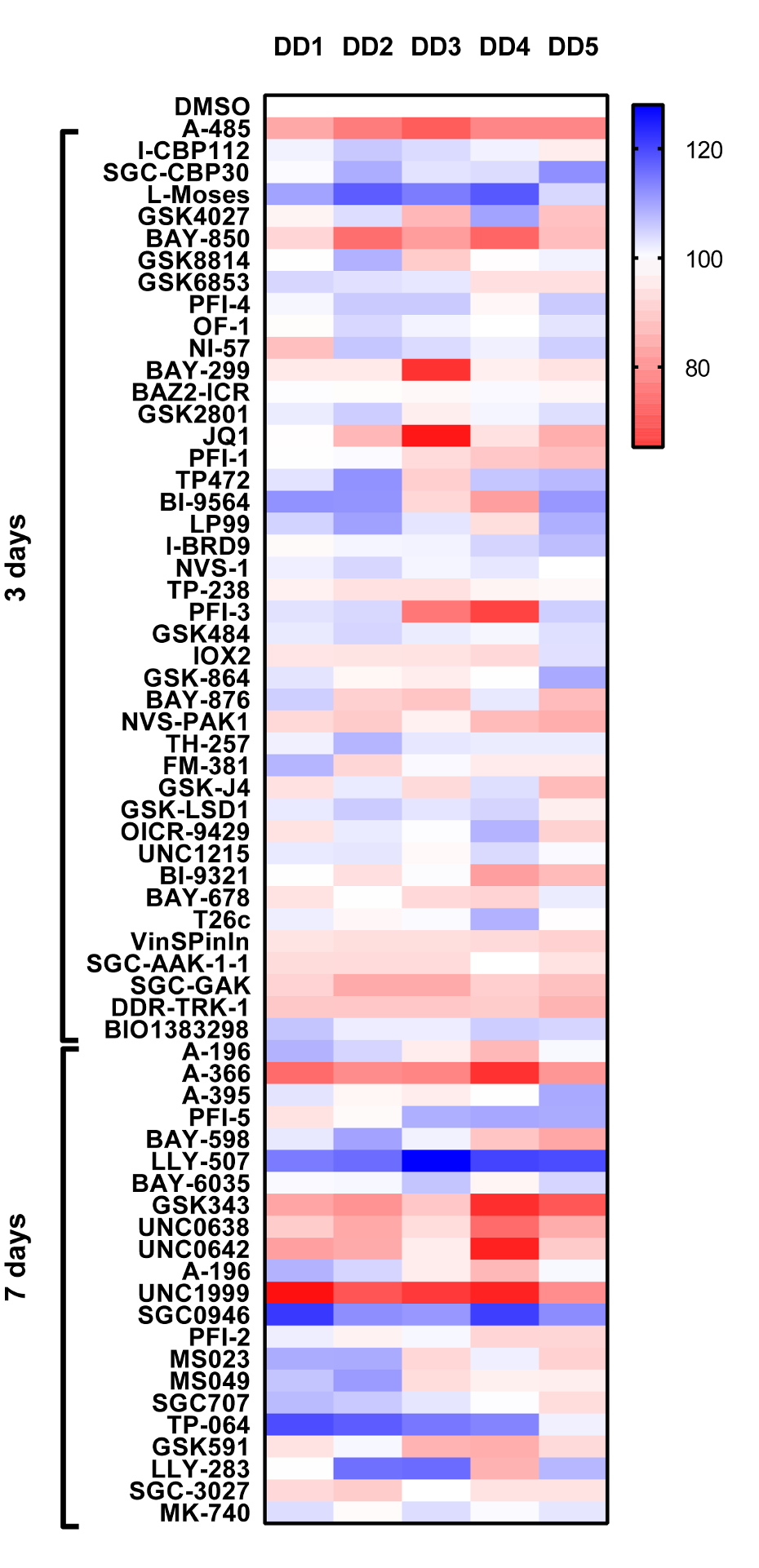
Analysing the cell counting/proliferation assay more probes seemed to display anti proliferative effects than simply on cell viability alone. A485 also effected proliferation but now the bromodomain CBP/EP300 probes I-CBP112 and SGC-CBP30 showed a mild anti-proliferative effect. Analogous to the viability data BAY-850, BAY-299 and the BRD4 probes JQ1 and PFI-1 showed similar anti-proliferative effects. A much broader spectrum of the methyl transferase inhibitors effected proliferation, now as well as the A-366 (G9a/GLP), GSK343 (EXH2), UNC0638 (G9a/GLP) and UNC0642 (G9a/GLP) and UNC1999 (EXH2) probes effecting proliferation the PRMT5 (LLY-283 and GSK591) and PRMT7 (SGC-3027) probes also displayed an anti-proliferative phenotype.
PrestoBlue®-Viability
- Surgically excised tissue from Dupuytren’s patients was treated with collagenase to derive single cell suspensions; cells were passaged (p2) and are termed myofibroblasts.
- Cells were seeded in 96 wells at 2 x103/well and left to adhere overnight. The following day probes were added at 1µM in triplicate, using DMSO as the vehicle control. Probes were incubated with the cells for 3 days or in the case of methyltransferase (MT) probes 7 days (cells were treated with MT probes again at day 3). 24 hrs prior to the end of the assay period staurosporine was added as a positive control cytotoxic agent (0.5uM).
- 6 hrs prior to the end of the assay PrestoBlue® 10X reagent was added to microplate wells. Incubate at 37°C for 6 hrs.
- Read fluorescence at 590 nm. Data is expressed as % compared to DMSO control (100%), DD1-5 Dupuytrens’s disease patients.
Proliferation-Cell Counting
- Surgically excised tissue from Dupuytren’s patients was treated with collagenase to derive single cell suspensions; cells were passaged (p2) and are termed myofibroblasts.
- Cells were seeded in 96 wells at 2 x103/well and left to adhere overnight. The following day probes were added at 1µM in triplicate, using DMSO as the vehicle control. Probes were incubated with the cells for 3 days or in the case of methyltransferase (MT) probes 7 days (cells were treated with MT probes again at day 3). 24 hrs prior to the end of the assay period staurosporine was added as a positive control cytotoxic agent (0.5uM).
- 1 hr prior to the end of the assay, Hoechst 33342 (1µg/ml, final concentration) was added to microplate wells.
- Quantitative cell counting was performed using the Celigo image cytometer. Data is expressed as % compared to DMSO control (100%), DD1-5 Dupuytrens’s disease patients.
PrestoBlue
Cell Number