Macrophage pro-inflammatory cytokine assay
The IL-23/IL-17 axis is of central importance in Psoriatic arthritis (PsA) and Spondyloarthritis (SpA) such as ankylosing spondylitis (AS). Macrophages, dendritic cells, ILCs, and mucosal-associated invariant T cells (MAITs) contribute to elevated IL-23 levels found in the serum, inflamed gut, synovial fluid, entheses, and bone-derived cells, including the marrow. An increased production of pro-inflammatory cytokines in particular IL-23 and TNFα in response to Toll-like receptor (TLR) agonists, such as lipopolysaccharide (LPS) has been reported to be clinically relevant for AS (1). IL-23 has been shown to drive IL-17 production by pathogenic Th17 cells (CD4) and is implicated in multiple autoimmune and inflammatory diseases including AS. Moreover, the observation that TNF-α is overexpressed in sacroiliac joints provided a strong rationale for the research of TNF-α inhibitors (2).
References
- McGeachy J., Y.Chen, C. M.Tato, A.Laurence, B.Joyce-Shaikh, W. M.Blumenschein, T. K.McClanahan, J. J.O’Shea, D. J.Cua. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10: 314–324.
- Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38(4):499–505.
To identify new targets and pathways that regulate IL-23, IL-12 and TNF-α release by monocyte-differentiated macrophages from AS patients and healthy controls.
In the TNF screen, the most striking results were observed with the p38 MAPK kinase inhibitor skepinone-L, the MEK inhibitors SCH772984 and Trametinib and the ATK inhibitor MK-2206, consistent with published literature. The other result of note was by the BRPF1B and BRPF2 bromodomain inhibitors OF-1 and PFI-4 which showed significant levels of inhibition of TNF release. In the TNFα, IL-12 and IL-23 screens the most striking result was the inhibition of the type I protein arginine methyltransferase inhibitor MS023 (and to a lesser extent the PRMT5 inhibitor GSK591) strongly inhibited cytokine expression in all donors. This is a novel finding and dose response studies demonstrate sub-micromolar activity. The other notable finding in the IL-23 screen was the enhancing effect of the BET inhibitors JQ1 and PFI-1. Also of interest the p38 MAPK inhibitor skepinone-L seemed to demonstrate significantly (P < 0.05) more activity toward the AS patient samples above the healthy controls. In the IL-12 screen the CBP bromodomain probes I-CBP112 and SCG-CBP30, and the BRD1/TAF inhibitor BAY-299 also strongly enhanced IL-12 expression.
PBMCs were isolated from whole blood from AS patients or HD using Histopaque and rested overnight at 37oC 5% CO2. CD14+ monocytes were then isolated by Miltenyi Biotec positive selection kit. Isolated CD14+ were suspended in RPMI 1640 containing 10% FBS and glutamine (R10) at 0.5x 106 / ml cell density and plated out in 60 x 25mm mono plates.
Monocyte differentiation into macrophages:
- Monocytes were cultured in R10 medium supplemented GM-CSF (20ng/ml) and cultured for 6 days at 37o Medium (R10) + GM-CSF was re-supplemented at day 3. Cells were harvested at day 6 using 'Accutase' (1 ml per plate for 20 minutes at 37oC, then washed with 10 ml of R10 and collected after centrifugation for 10 min at 300Xg).
- Macrophages were plated at 2 x 104 cells / well and left a further 3 days with or without the addition of compounds (1µM final concentration or DMSO control).
- At the end of the 3 days, wells were stimulated with 20ng/ml LPS, 20ng/ml IFN-γ and left for 24 hours.
- Supernatants were harvested at the end of the stimulation period and assessed for IL-12 and IL-23 and TNFα production
- AS (AS prefix) patient cells or HC (Healthy control donor) were cultured according to the described protocol and IL-23, IL-12 or TNFα production was measured in the culture supernatants by ELISA. Results are normalized to the negative DMSO control for each donor.
IL-12
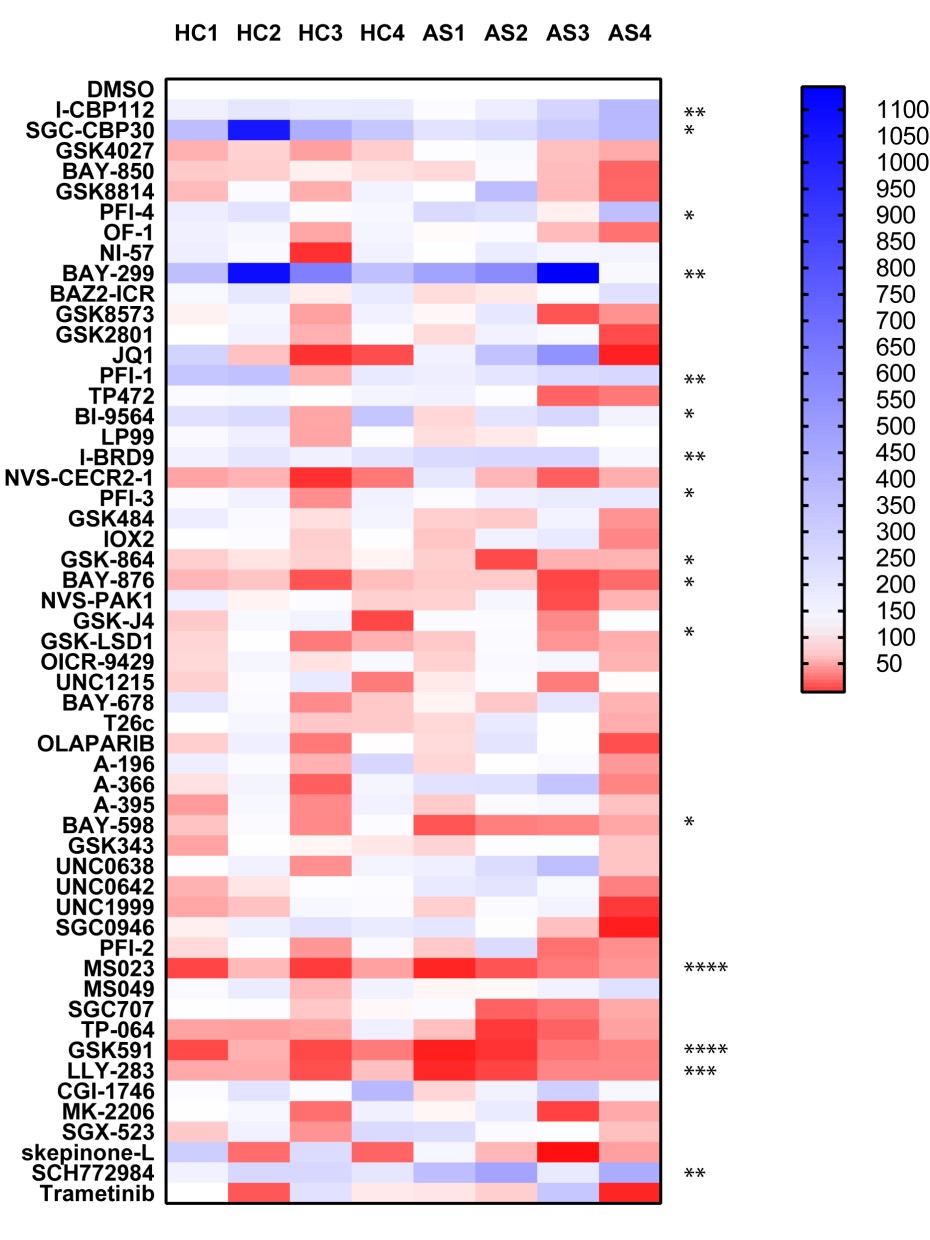
P value was determined by one sample t test to normalised control (DMSO) sample of 1.
P *<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
IL-23
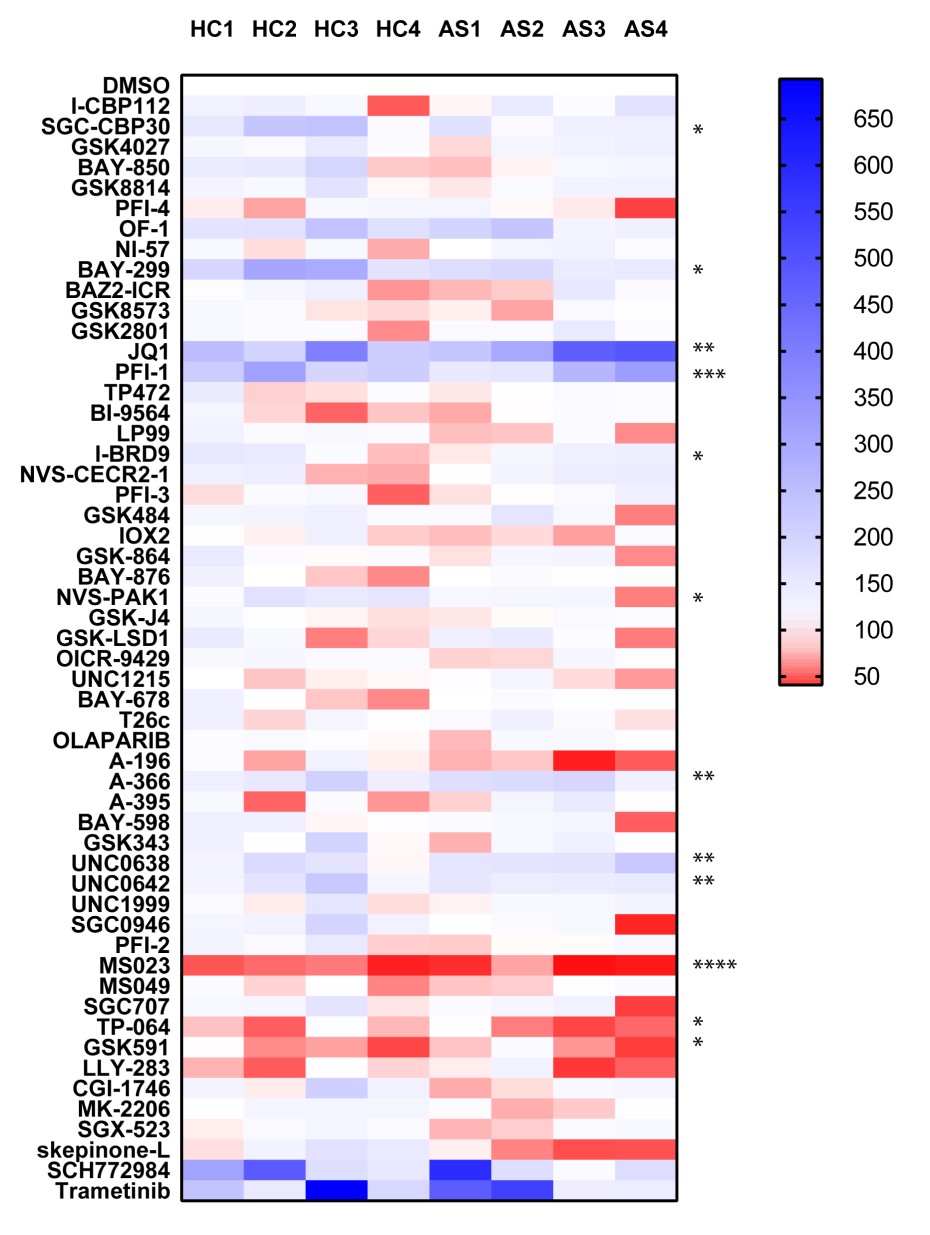
P value was determined by one sample t test to normalised control (DMSO) sample of 1.
P *<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
TNF
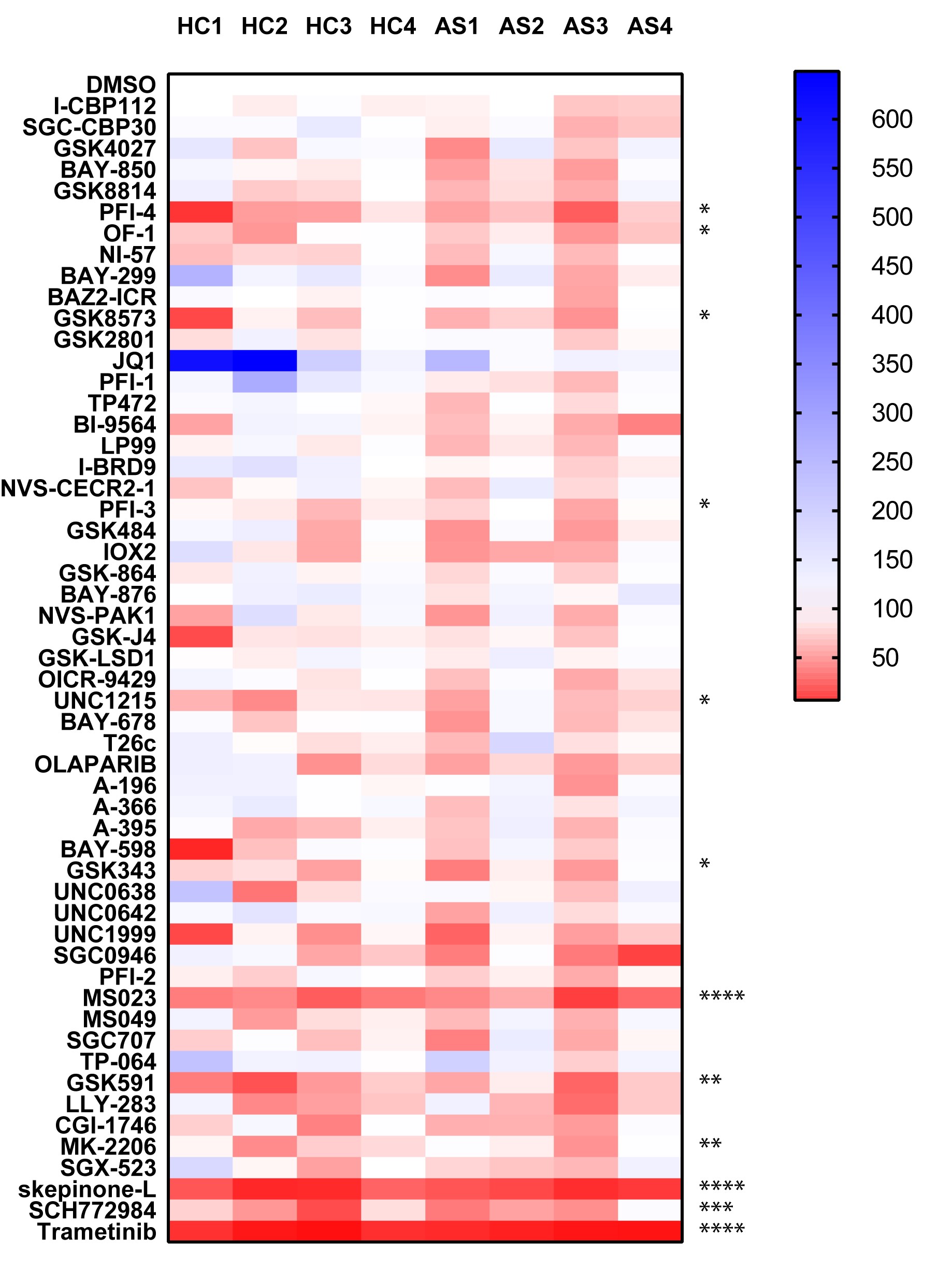
P value was determined by one sample t test to normalised control (DMSO) sample of 1.
P *<0.05, ** P<0.01, *** P<0.001, **** P<0.0001
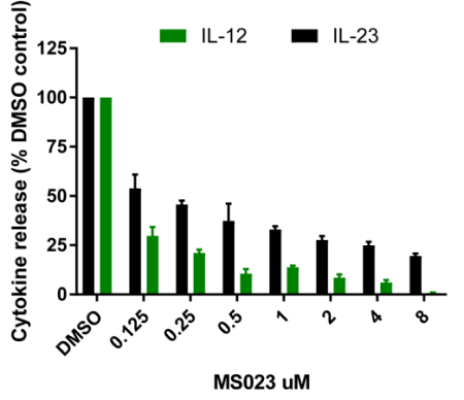
Data is expressed as % compared to DMSO control (100%) mean +/- SEM of 3 donors