3D map of PC2 to jump-start drug discovery efforts in polycystic kidney disease
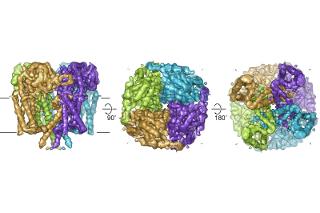
Oxford, UK, December 19, 2016, Scientists from the Structural Genomics Consortium (SGC) and Oxford University report a three-dimensional view of the Polycystin-2 (PC2), a protein that causes autosomal dominant polycystic kidney disease (ADPKD) when mutated, in an article published today in Nature Structural and Molecular Biology.
ADPKD is one of the most common life-threatening genetic diseases, affecting ~1 in 800 individuals at birth. The disease is characterized by the formation of fluid-filled cysts that compromise kidney function and cause substantial pain for patients. 50% of patients with ADPKD eventually develop end-stage kidney disease and require dialysis or a transplant to survive.
Mutations in the genes that encode polycystin-1 (PC1) and PC2 cause the disease, but the mechanisms by which these mutations disrupt the function of these proteins and lead to disease are not known. PC1 and PC2 proteins are embedded in multiple membranes of kidney cells, regulate the movement of positively charged ions into these cells or into specialized compartments within the cells, and impact cellular signaling.
Scientists led by Profs Liz Carpenter and Juha Huiskonen at the University of Oxford employed a technique called cryo electron microscopy (cryoEM) to provide a three-dimensional view of PC2. The work revealed a novel substructure called the Tetragonal Opening for Polycystins or TOP domain, which caps the protein. At least 27 of the known ADPKD-causing mutations map to this region of the protein, and this structure provides important insight into how the TOP domain impacts the ability of PC2 to bind to and control the movement of ions.
“There is a significant need for scientists to provide insight into the biology of PC1 and PC2 so we can develop medicines to help patients with ADPKD. These structural insights will help scientists begin to understand how mutations in PC2 cause disease, and more importantly, generate ideas for how the disease can be treated,” said Chas Bountra, Director of SGC Oxford.
SGC scientists now plan to identify small molecules that stabilize PC2, with the hope of supporting its function in kidney cells and inhibiting cyst formation.
The paper, Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2), was published December 19th online in Nature Structural and Molecular Biology.



